Basics of the Biological Nutrient Removal
Process
- To Introduce the reader to the basic framework of the Biological Nutrient Removal (BNR) Process.
- To provide basic guidance in using this basic framework in optimizing and troubleshooting BNR processes.
Learn Theory! Learn Theory!
Learn Theory! If you know theory you can use it! If you don’t know theory you
can’t use it!
Thinking in terms of complex
systems is difficult. Many people, even after studying for years, do not
understand basic principles. Systems thinking is often counterintuitive at many
points. It takes many years to gain any degree of skill, and it still often
eludes us. (Extraordinary Leadership: Thinking Systems, Making a
Difference, 2009, Reberta M. Gilbert, M.D, pg 5)
This article is not a primer or
refresher on nitrification, denitrification or phosphorous removal. It assumes
the reader already has a basic understanding of these processes. If you feel
you need a refresher, please refer to any of the Water Environment Federation’s
(WEF) Manual of Practices (MOP) for Wastewater. A copy of MOP 11 can be found
for free download on the internet. Key concepts crucial for understanding the
BNR process will be provided at specific places in the discussion.
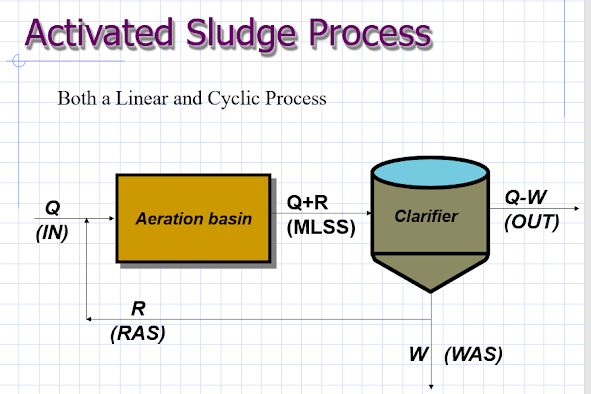
For example, it is important to
remember that the activated sludge is a cyclic process as well as linear one.
The fact that each tank has an influent and an effluent is a sufficient
reminder of the linear aspect of the process. What is more difficult to remember
is the cyclic aspect of the process. Even the most basic activated sludge
process has a sludge recycle (see Figure 1).
FIGURE 1
The purpose of any
recycle is to return a portion of the waste stream from one part of the process
to another part of the process. The return sludge in a conventional activated
sludge process ensures that the settled biomass is recycled back to the head of
the aeration basin. Without this recycle, the aeration basin would never
develop a large enough biomass to remove the substrate (BOD, phosphorous or
nitrogen).
Interestingly, the most basic BNR
process, and one of the first activated sludge processes developed early on,
did not have any recycle. That process is the Sequencing Batch Reactor (SBR).
In the BNR mode, wastewater enters the tank at the beginning of the process
(Mix/Fill) while the MLSS is in an anaerobic condition, then aeration begins
(React/Fill) placing the MLSS in an aerobic condition, after which the filling
stops (React On/Off). When the MLSS is almost fully oxidized the next two phases
of the SBR process begins (Settle), and finally (Decant/Idle). The MLSS is in an
anoxic condition at this point. Then the process starts over again. Since it is
a batch process and everything proceeds sequentially in a single tank, there is
no need for recycle (Figure 2).
FIGURE 2
The beauty of the SBR process is the BNR process follows the logical sequence required by nature. For this reason, it is the perfect place to begin learning about the BNR process. Figure 3 illustrates the basic stages of the BNR process. The anaerobic section (Mix/Fill) of the SBR process is Stage One of the luxury phosphorous uptake process. The aerobic section (React/Fill & React) of the SBR represent Stage Two of the luxury phosphorous uptake process and Stage One of the Nitrogen Removal Process (Nitrification). The anoxic (Settling and Decant) sections represent Stage Two of the Nitrogen Removal Process (Denitrification). Carbonaceous BOD removal occurs throughout the whole SBR process.FIGURE 3

Included in Figure 3 is a secret
that many operators are not aware of. Optimum BNR occurs only if the wastewater
remains in each of the three major respiratory modes (anaerobic, aerobic and
anoxic) long enough to complete the process. If the Mix/Fill phase is not long
enough, the first stage of the luxury phosphorous uptake process (phosphorous
release) will not be complete. The same is true for stage two of the luxury
phosphorous uptake process (phosphorous uptake), nitrification, and
denitrification. The percentages located below the three respiration modes
represent the relative amount of the total treatment time required for that
particular mode. For example, stage one of luxury phosphorous uptake requires
somewhere between 15 to 20% of the total treatment time in the SBR process.
Another way to visualize it is represented in Figure 4.
FIGURE 4
Why is this important?
When SBRs have difficulty with
phosphorous, ammonia or nitrate removal, in many cases it is due to a
deficiency in treatment time in one of the three respiration modes. An increase
in effluent phosphorous can often be traced back to either 1) not enough time
in the anaerobic mode or 2) not enough time in the anoxic mode. 1) can be
corrected by lengthening the Mix/Fill phase. 2) results in incomplete
denitrification during the Settling/Decant phase which allows nitrates to enter
the Mix/Fill phase of the SBR process which is supposed to be “anaerobic.” The
reason for the quotes is, as we all know, “anaerobic” is defined as “zero DO,
zero nitrates”. If nitrates are in the “anaerobic” phase of the process, that
phase is not anaerobic, but anoxic, no matter what we may call it. In the
second scenario either a) the settle/decant phase is not long enough, b) the DO
is too high at the end of the aerobic (react) phase, or c) the (React) phase is
too long.
Just remember that when making
changes to the time of any one of the phases it will affect the times of the
other phases as well.
What Happens in Each of the
Three Respiration Modes?
Figure 5 illustrates the relative
concentrations of BOD, phosphorous, ammonia, nitrate/nitrite and alkalinity
levels during the anaerobic, aerobic and anoxic modes.
FIGURE 5 Relative Nutrient Concentration During the BNR Process
Under normal conditions the
phosphate concentration goes up during this period, often as much as four times
the influent concentration. During the aerobic period of the process the PAOs will
take up as much as seven times the amount of PO4 they originally released. The PO4
concentrations at the end of the anaerobic period should be below 0.5 mg/L. Tracking
the PO4 concentrations at the influent and effluent end of the anaerobic
period is a good indicator of the health and effectiveness of the PAOs.
Nitrogen is found in the influent
in two primary forms; approximately 40% is in the form of ammonia (NH3)
and approximately 60% is in the form of complex organic nitrogen measured as
Total Kjeldahl Nitrogen (TKN). The TKN portion of the nitrogen is converted to
ammonia during the anaerobic and aerobic periods of the BNR process. Thus, if
just ammonia is measured in the influent at a concentration of 12 mg/L, the
amount of TKN could be around 30 mg/L. The actual ammonia that would have to be
converted is around 40 – 45 mg/L. This is reflected by the rising yellow line during
the aerobic period.
During the aerobic period the
nitrifiers are actively converting ammonia to nitrite/nitrate (NOX).
This is represented by the falling yellow line (NH3) and rising
purple line (NOX) during the aerobic period. By the end of that
period the NH3 should be well below 1.0 mg/L and the NOX
should be in the 30 – 40 mg/L range.
During the anoxic period of
treatment, nitrate/nitrite (NOX) will be converted to nitrogen gas
(Ná). It is important that as much (NOX)
be converted to (Ná)
as possible before the process is returned to the “anaerobic” period of
treatment. Any (NOX) entering the “anaerobic” period shortens that
period. Remember, (NOX)’s in the MLSS means it is still anoxic.
If the BNR process is working
properly, the alkalinity (and eventually the pH) will decline during the
aerobic period due to the nitrifying organisms (remember they are autotrophic –
they consume inorganic carbon such as carbonate). During the anoxic phase, however,
the denitrifying bacteria will recover approximately 50% of the original
alkalinity.
Process Control
So, based on the above discussion,
it should be pretty obvious what process control samples need to be pulled and
where. Figure 6 illustrates the process control samples that should be pulled
for optimizing and monitoring the process. The picture includes a clarifier and
internal recycle for a system that is not an SBR, but the principles are the same.
FIGURE 6 Putting It All Together
The primary different between sampling
and testing for process control and troubleshooting is frequency. When
everything is working well the amount of sampling can be greatly curtailed.
However, if the system is experiencing problems, the type and severity of
problem, will dictate the location, frequency and duration of the sampling and
testing. For example, it is not necessary to test for ammonia if the system is experiencing
elevated PO4 values.
Feel free to contact me should
you serve a population >10,000 or have any questions or issues that the GRWA
is unable to assist you with. Also, if you would like to contribute an article
feel free to email me at the address below. I am always looking for
contributors that have an interesting perspective, topic or has an interesting
case that they would like to share – especially if the solution is a direct
result of applying the principles from this forum.
Dennis Brown,
Wastewater Specialist and Trainer, Retired
678.750.3996





Comments
Post a Comment